As we continue to navigate the evolving landscape of pharmaceutical production, lyophilization stays an indispensable Resource that safeguards the potency and stability of pharmaceutical products together with biologic products for patients.
For that reason the product needs to be packed in vacuum or making use of inert gasoline or in a container impervious to gases Price tag could be a problem, according to the products While process
This video clip demonstrates how the FreeZone Triad Benchtop Freeze Dryer is built to lyophilize the widest assortment of sample forms: from bulk things, vials and microplates on heated cabinets to substantial samples in flasks.
Controlled freezing prices, together with annealing and process knowledge of supercooling outcomes, are sometimes employed to accomplish uniform ice crystal distribution. Newer systems will also be giving the chance to nucleate on demand, even more raising products uniformity across lyophilizer shelves, which is a highlight in foreseeable future lyophilization engineering.
Think of These light-weight, nonetheless flavor-packed freeze-dried berries within your cereal or the instant coffee that wakes you up each morning.
This is a preview of subscription content, log in by means of an institution to examine entry. Access this ebook
Cabinets: They supply the floor on which the product or service rests and will be cooled or heated to regulate the freezing and drying processes.
With yrs of expertise within the pharmaceutical industry, we know exactly what a great infrastructure check here for the cryogenic freeze dryer desires.
A prosperous lyophilization cycle can manage the Essential Excellent Attributes (CQAs) on the item throughout the merchandise lifecycle with least time and Strength use. Down below are some essential results aspects:
Sample storage: refrigeration units and applications Storing Organic factors almost indefinitely, without any change or degradation in cells. That's the aim of sample
A different review located that 60% of child foods in the U.S. You should not meet up lyophilization pharmaceutical products with nutritional tips established by the whole world Health and fitness Firm (WHO). Numerous foods were being lower…
Inside the secondary or remaining drying section, the residual dampness material is decreased as much as is possible to make certain that the product or service is inside of a forever storable condition. The h2o sure by adsorption at The interior surface area of the item has to be taken off. To accomplish this, it is frequently required to defeat h2o’s capillary forces.
Primary Drying (Sublimation): All through this stage, tension is manipulated to convert water straight from strong to gas through sublimation, as well as the ensuing h2o vapor is gathered on the condenser.
The cookie is set by the GDPR Cookie Consent plugin and is utilized to retail store if consumer has consented to the use of cookies. It doesn't shop any personal facts.
 Kel Mitchell Then & Now!
Kel Mitchell Then & Now! Neve Campbell Then & Now!
Neve Campbell Then & Now! Rick Moranis Then & Now!
Rick Moranis Then & Now! Robbie Rist Then & Now!
Robbie Rist Then & Now!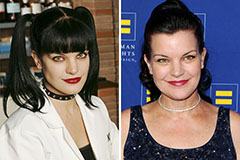 Pauley Perrette Then & Now!
Pauley Perrette Then & Now!